United Living Colors of Phelsuma lizards


United Living Colors of Phelsuma lizards




Back to the
“LANE”
introduction page

PUBLICATIONS
‣BMC Biology 2013, 11: 105

!! CHECK ALSO !!

PEOPLE INVOLVED
FROM MICHEL
MILINKOVITCH’S LAB
‣Suzanne Saenko

MAIN COLLABORATORS
Jérémie Teyssier & Dirk van der Marel
Quantum Materials Group,
Dept of Physics,
University of Geneva,
Switzerland


Colors of lizards: as much Physics as Biology
Combining Physics and Biology techniques (histology, optics, mass spectrometry, UV and Raman spectroscopy, mathematical modeling), we show that the extensive variation of skin color and patterns in Phelsuma lizards is generated by precise co-localization of interacting pigmentary and nano-structural elements (Saenko et al. BMC Biology 2013, 11: 105). More specifically, we show that:
(i)the vivid blue and/or green color of dorso-lateral skin is modulated both by variation in the geometry of highly-ordered intracellular nano-crystals (generating a very specific and very intense color) and by the presence of yellow pigments;
(ii)the reflectivity of the white belly and of dorso-lateral pigmentary red marks is increased by underlying disorganized nano-crystals forming broadband reflectors.
These interactions require precise co-localization of yellow and red chromatophores with different types of iridophores, characterized by ordered and disordered nanocrystals, respectively. We validated these results through numerical simulations combining pigmentary components with a multilayer interferential optical model. Finally, we show that melanophores form dark lateral patterns but do not significantly contribute to variation in blue/green or red coloration, and that changes in the pH- or redox-state of pigments provide yet an additional source of color variation in squamates. This study opens up new perspectives for Phelsuma lizards as models in Evolutionary Developmental Biology and Physics of Biology.
This multidisciplinary project has been performed in collaboration with the lab of Dirk van der Marel (Quantum Materials Group, UniGE) and is part of two research consortia:
➡The ‘United-Living-Colors.ch’ consortium (funded by the Swiss National Foundation SINERGIA program) investigating the complex interactions between light and the vertebrate skin and how they generate a variety of phenomena of significant fundamental and applied interest;
➡The ‘EpiPhysX’ consortium (funded by SystemsX.ch), with the aim to understand how epithelial tissues achieve their complexity.
See our ‘Physics of Biology’ page.
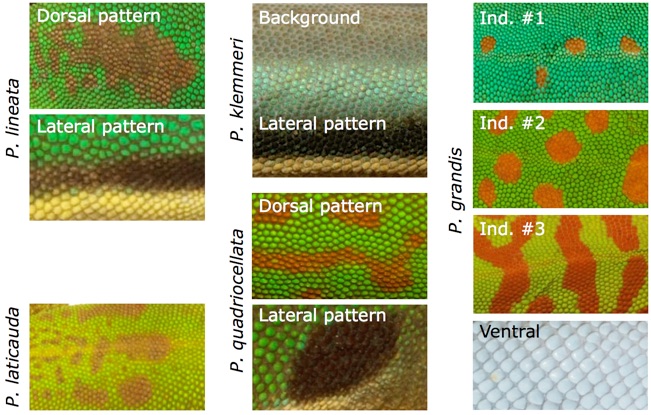
Colour and pattern variation in the genus Phelsuma
The 46 currently recognized species of Phelsuma exhibit a range of background coloration, as well as dorsal and lateral patterns. Species indicated in bold were used in this study because their coloration represent variation found across the genus. The skin on the belly (is off-white in the majority of the species. See Saenko et al. BMC Biology 2013, 11: 105 for much additional details.
Pigmentary and structural colorations in Phelsuma geckos
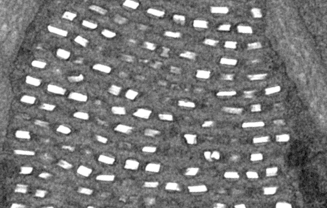
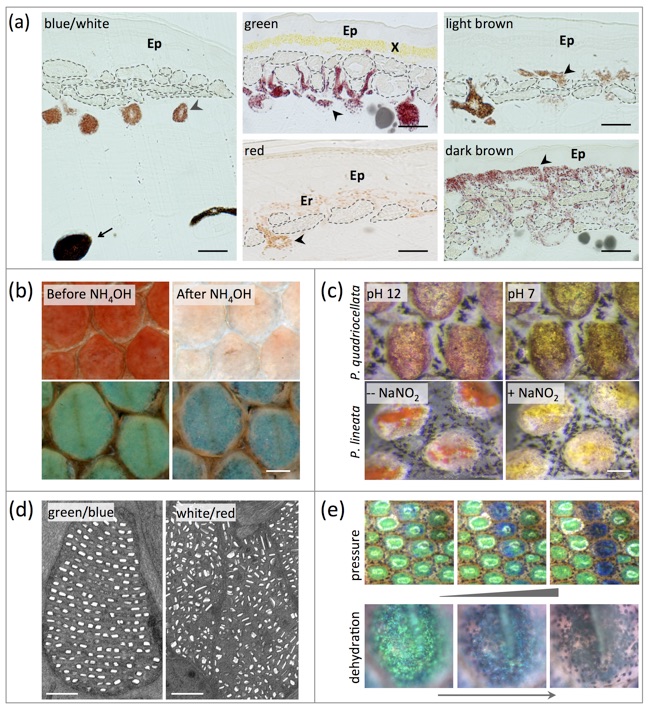
Structural colors are generated in iridophore cells by constructive interference of light with guanine nanocrystals. (a) Histological analyses identify two types of melanophores, iridophores, xanthophores, and erythrophores. (b) Pteridin pigments are removed with NH4OH, revealing the remaining structural color produced by iridophores. (c) Red pigments in dorsal markings can change color when the pH is lowered or when an oxidant is added. (d) Iridophores in green/blue and white/red skin have respectively organized or disorganized guanine crystals. (e) Mechanical pressure and dehydration (see also the two movies below) lead to a blue shift of structural green. See Saenko et al. BMC Biology 2013, 11: 105 for much additional details.
Click on the images below to see the supplementary movies:
Left, Real-time movie showing how a pressure manually applied with tweezers locally modifies the structural color (by reducing the period of the photonic crystal) and how the relaxation process drives the skin back to its original color. Right, Accelerated movie (16x) showing the effect of dehydration and re-hydration on the structural color generated by iridophores. In both movies, the yellow pigments have been removed prior to the experiment.
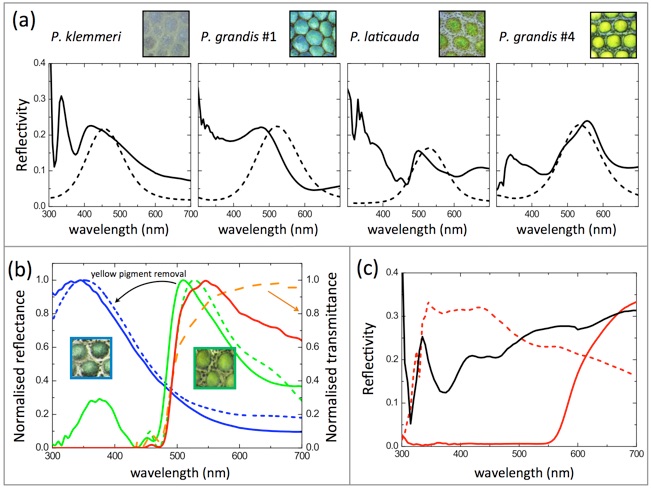
Experimental and modeled reflectivities of Phelsuma skin
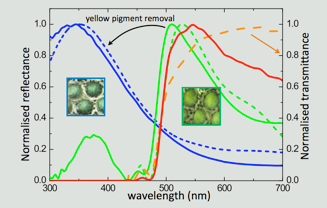
(a) Measured skin reflectivities (solid lines) after removal of pigments compared to modelled reflectivities (dashed lines). The mathematica model is described in the Supplementary File 2 of the original paper. (b) Normalized reflectivity of green skin before and after yellow pigment removal (green and blue solid lines, respectively). Multilayer responses are also shown without (dashed blue line) and with (dashed green line) a 3 μm thick pigment layer on top. The direct product of structural blue reflectivity with normalized yellow pigment transmittance generates the dashed red line, confirming the mechanism of structural color filtering by the top pigment layer. (c) Reflectivity measured on white skin (black line), as well as red skin before (red solid line) and after (red dashed line) red pigment removal. Reflectivity intensities of ordered and disordered iridophores are comparable. See Saenko et al. BMC Biology 2013, 11: 105 for much additional details.
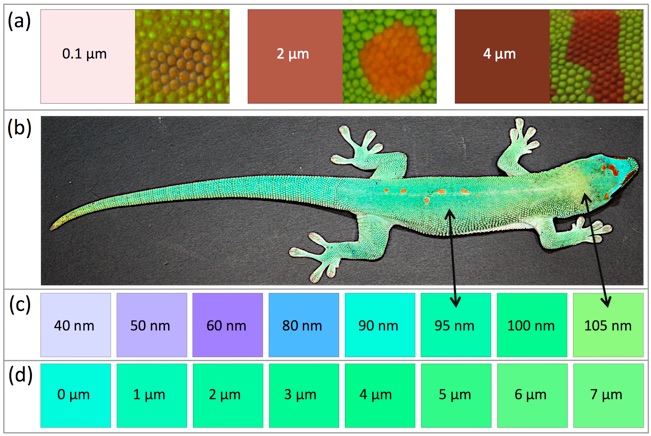
Colors simulated with the multilayer model.
(a) Red colors simulated with varying thickness (0.1 to 4 µm) of a red pigment layer on top of a white reflector and comparison to red markings of different animals. (b) P. grandis individual. (c) Simulated colors produced by a 7 µm yellow pigment layer on top of a multilayer interference reflector with varying spacing (40 to 105 nm) among layers of 80nm-thick crystals. (d) Simulated colors produced with varying thickness (0 to 7µm) of a yellow pigment layer on top of a blue reflector.
Conclusions and perspectives
Extensive variation in background coloration, as well as in dorsal and lateral color patterns, is observed within and among species of the genus Phelsuma. Our analyses indicate that this variation is generated by a combination of features associated to pigmentary and structural-color chromatophores. Structural colors are generated by constructive interference of light with guanine nanocrystals. Blue skin color is solely due to iridophores with well-organized guanine nanocrystals (i.e. narrow-band multilayer interference reflectors), whereas green skin is produced, depending on the species and the individual, either by structural green or by the interaction of structural blue with yellow pigments (xanthophores). Dorsal marks are formed by red erythrophores, the reflectivity of which is enhanced by iridophores with disorganised crystals (i.e. broadband reflectors producing incoherent scattering). In addition, the hue produced by erythrophore and xanthophore pigments is pH- or redox-dependent in some species. Most importantly, we show that the colour patterns of Phelsuma always require precise co-localization of different sets of interacting pigmentary and structural elements. For example, yellow and red chromatophores are associated to iridophores with ordered and disordered nanocrystals, respectively. This indicates the need to identify the developmental mechanisms responsible for the superposition of specific chromatophore types, opening up new perspectives for Phelsuma lizards as model organisms in evolutionary developmental biology.
Publications
Please, consult the full publications below for references & much additional information.
✓Saenko S., Teyssier J., van der Marel D. & M. C. Milinkovitch
Precise colocalization of interacting structural and pigmentary elements generates extensive color pattern variation in Phelsuma lizards
BMC Biology 2013, 11: 105
Other related publications
Coverage
➡Check Q&A in Biome: online highlights from BioMed Central journals
➡Check coverage (TV programs, Websites, and News Papers) HERE