Galápagos giant tortoises


Galápagos giant tortoises




Back to the
“Conservation Genetics” page
Back to the
“LANE”
introduction page


FACTS
Species:
‣Geochelone nigra
Locations:
‣Galápagos Islands

PUBLICATIONS
‣Molecular Ecology, 11: 2265-2283 (2002)
‣Animal Conservation, 6: 329 -337 (2003)
‣Proceedings of the Royal Society, London, B, 271: 341-345 (2004)
‣BMC Ecology, 7:2 (2007)
‣Chapter 12 (pages 269-293) In “Population Genetics for Animal Conservation”, Cambridge University Press (2009)
‣Check also Nature, 429: 498-500 (2004)

Related publications from our group on population genetics
‣Check the main “Conservation Genetics” page

PEOPLE INVOLVED
FROM MICHEL
MILINKOVITCH’S LAB
‣Daniel Monteyne

MAIN COLLABORATORS IN THE GALAPAGOS
Charles Darwin Foundation (CDF)
The CDF has carried out research for the conservation of the Galápagos for 50 years.
&
Galápagos National Park Service (GNPS)
The mission of the GNPS is to protect, conserve and manage the Archipelago ecosystems and its biological diversity.
People at both the CDF and GNPS have been pivotal by providing their technical, logistic, and scientific expertise.
The main people involved are:
✓James Gibbs (CDF, & see below)
✓Thomas H. Fritts (CDF)
✓Howard L. Snell (CDF & University of New Mexico - web page)
✓CruzMarquez (CDF)
✓Washington Tapia (GNPS)

THE YALE TEAM
A large proportion of the Galápagos giant tortoise project has been carried out at Yale University. The people involved are:
Jeff Powell
Professor at Yale University, Jeff is the initiator of the conservation genetics project on Galápagos giant tortoises.
Gisella Caccone
Is the leading investigator on many of the papers on the conservation genetics of Galápagos giant tortoises.
Michael Russello
Was post-doc in Jeff & Gisella’s labs. He is now at the University of British Columbia, Canada.
Claudio Ciofi
Was post-doc in Jeff & Gisella’s labs. He is now Associate Prof at the University of Florence, Italy.
Luciano Beheregaray
Was post-doc in Jeff & Gisella’s lab. He is now Associate Prof at the Macquarie University, Sydney, Australia.

OTHER PEOPLE INVOLVED
James Gibbs
Professor in Conservation Biology & Wildlife Management at the State University of New York & Adjunct faculty member at the Charles Darwin Foundation (Galápagos islands). James has been actively involved since the beginning of the conservation genetics project on the Galápagos tortoises.
Ralph Tiedemann
Was a Post-doc in Michel Milinkovitch’s lab. He is now Full Professor at the University of Potsdam, Germany.






Population Genetics and Conservation of the Galápagos tortoises
The Galápagos tortoise (Geochelone nigra) is the largest living tortoise (adults can weigh over 300 kg) and it can live more than 150 years. Populations declined dramatically since the 17th century because of poaching as well as habitat destruction by feral species (goats, dogs, rats, ...). Today three island populations are extinct, only one individual (the famous “Solitario Jorge” or “Londsome Georges”) survives from the island of Pinta, and several populations are critically endangered.
A large, international conservation genetics project on the Galápagos Giant Tortoise has been ongoing since 1995 (largely lead by Jeffrey R. Powell and Gisella Caccone, Yale University). Our responsibility included the isolation, characterization, and analysis of species-specific microsatellite loci.
Below, we will focus on the subproject in which we have most contributed: the analysis and assessment of the particularly spectacular captive breeding and reintroduction program of the Española population.
Please, consult the full publications below for references and much additional information on our conservation genetics work on Galápagos tortoises:
✓Ciofi C., Milinkovitch M. C., Gibbs J.P., Caccone A. & J. R. Powell
Microsatellite analysis of genetic divergence among populations of giant Galápagos tortoises
Molecular Ecology, 11: 2265-2283 (2002)
✓Burns C.E., Ciofi C., Beheregaray L.B., Fritts T.H., Gibbs J.P., Márquez C., M. C. Milinkovitch, Powell J.R. & A. Caccone
The origin of captive Galápagos tortoises based on DNA analysis: Implications for the management of natural populations
Animal Conservation, 6: 329 – 337 (2003)
✓Milinkovitch M.C., Monteyne D., Gibbs J.P., Fritts T.H., Tapia W., Snell H.L., Tiedemann R., Caccone A. & J. R. Powell
Genetic analysis of a successful repatriation program: Giant Galápagos tortoises
Proceedings of the Royal Society, London, B, 271: 341-345 (2004)
✓Check also “News Feature: One of a kind” by H. Nicholls”
Nature, 429: 498-500 (2004)
✓Milinkovitch M.C., Monteyne D., Russello M., Gibbs J.P., Snell H.L., Tapia W., Marquez C., Caccone A. & J. R. Powell
Giant Galápagos Tortoises: Molecular Genetic Analysis Reveals Contamination in a Repatriation Program of an Endangered Taxon
BMC Ecology, 7:2 (2007)
✓Ciofi C., Caccone A., Beheregaray L.B., Milinkovitch, M.C., Russello M., & J.R. Powell
Genetics and conservation on islands: the Galápagos giant tortoise as a case study
Chapter 12 in “Population Genetics for Animal Conservation” (Bertorelle G, Bruford M.W., Hauffe H.C., Rizzoli A., & Vernesi C., eds.), Cambridge University Press 2009
History of the near extinction of the Española tortoises
When natural populations of endangered species shrink to the point of no longer being self-sustaining, as a last resort to avoid outright extinction, remaining individuals may be brought into captivity. If the species is able to reproduce in captive settings and if the factor(s) causing the population decline in nature can be ameliorated, subsequent release of offspring can potentially restore a self-sustaining population in the original natural habitat. Rarely has it been documented what proportion of the genetic diversity of the breeders is actually represented in the repatriated offspring in nature. Because captive breeding and population restoration underpin many conservation efforts around the world, detailed assessments are needed of how genetic diversity changes through each of the phases of near extinction, captive breeding and population restoration.
Española Island in the Galápagos Archipelago is the site of one of the most successful, but least heralded, species reintroduction efforts ever attempted. Once numbering at least 3000, tortoises on Española had been reduced, by 1965, to just 14 individuals by hunters from sealing, whaling and pirate ships (Española is one of the flattest and most accessible islands in the Galápagos Archipelago). Whalers also introduced goats to the island, which over the course of 200 years dramatically increased in numbers and converted a densely vegetated island to one of open thorn scrub. With no apparent tortoise reproduction taking place by the mid-1960s, the remaining 14 tortoises (two males and 12 females) were transferred to the Breeding Centre of the Charles Darwin Research Station and Galápagos National Park on Isla Santa Cruz. During the 1970s, goats were eliminated from Española as the result of an intense campaign by the Galápagos National Park Service, setting the stage for ecological restoration of the island. The first tortoises, whose parents originated from Española, hatched in 1971 and were subsequently repatriated in 1975. In 1977, the number of males was augmented by the arrival of a third adult male from the San Diego Zoo. This core population of 15 individuals continues to reproduce in captivity at the Breeding Centre and by August 2002 had generated 1200 repatriated offspring.
Much of the vegetation (except the endemic Opuntia cactus) has recuperated rapidly on Española since the elimination of feral goats. Repatriated tortoises are now reproducing in situ: in 1988 tortoise nests were observed on Española, and offspring hatched in the wild were documented in 1994.
First molecular genetic analysis of the Española breeding program
Reproduction by the released individuals is a good indication that this repatriation programme may be on its way to achieving a healthy self-sustaining population in the original rehabilitated habitat. However, because little effort was made to match pairings of male and female breeders, little is known about how much of the genetic diversity of the 15 breeders is represented in the repatriated population. We first used microsatellite DNA markers to determine the maternity and paternity of 134 repatriated offspring and to estimate the impact of the current breeding-programme settings on the genetic effective population size (Ne), probably a major determinant of the long-term survival of the repatriated population.
Note: We computed effective population sizes of females and males taking into account the observed variance in reproductive success. The effective population size is the number of breeding individuals in an idealized population that would show the same amount of inbreeding as the population under consideration. When there is a biased sex ratio, the effective population size is reduced following:
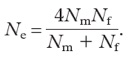
where Nf and Nm are census numbers of females and males, respectively.
The effective population size is further reduced if different individuals exhibit different reproductive success. In that case, Ne of females and males (Nef & Nem) can be computed as follows:
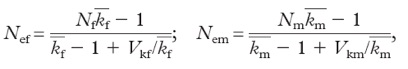
where kf and km are the means of offspring number per female and male breeder, respectively, and Vkf and Vkm are the variances in offspring number per female and male breeder, respectively.
Results
We extracted DNA and screened the Española breeders for variation at 69 microsatellite loci to find 15 loci informative for maternity and paternity analysis. This population is exceptionally low in genetic variation, having: (i) a single maternal lineage (as assessed by mitochondrial DNA); (ii) an average of 2.7 alleles per microsatellite locus; and (iii) a mean expected heterozygosity (He) per locus of 0.537. These numbers are much lower than those exhibited by other Galápagos tortoise populations. Out of the 134 offspring analysed, only two could not be assigned to parents.
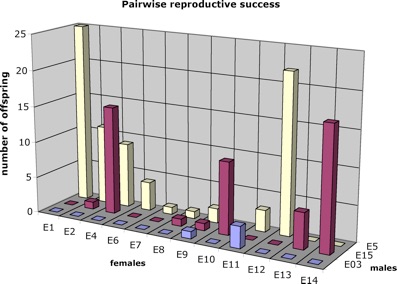
These very unequal contributions among parents along with unequal sex ratio of the breeding population lead to a very reduced Ne of 7.6 and 1.8 for females and males, respectively, leading to an overall Ne of 5.7. Because the long-term Ne is the harmonic mean across generations, this very low Ne in the breeding population will have a long-term effect on the Ne of the repatriated population. For example, even if the F1 repatriated population had an Ne of 1000, the Ne over the two generations would be 11.3; if Ne remains at 1000 for 10 generations, long-term Ne reaches only about 59. Rectifying the low Ne in the parents would have a long-term effect on the repatriated population. An easy way to increase variability in the repatriated population is to equalize reproductive success among breeders. Indeed, although this breeding scheme might not fully maximize diversity (because it assumes that breeders are ‘unrelated’), it will certainly yield a higher genetic diversity than that generated under the strongly skewed distribution observed here.
Unequal contributions among parent individuals could be the result of four factors or, most likely, a combination of them. First, not all pairs had equal opportunity to mate. In the breeding programme, males and females were moved infrequently between two pens, so not all males had equal access to all females. Second, there may be nonrandom mating occurring; given the limited movement of tortoises and the potential for social dominance in pens with more than one male, it is not clear how to disentangle this from the first point. Third, fertility may vary greatly. For example, male E3’s low contribution may reflect low fertility; likewise female E7 may be less fecund than the other females. On the other hand, females E1, E10, E12 and E14 are clearly highly fecund, but are producing repatriated survivors with only one male. Finally, the unevenness of genetic representation may reflect differential survivorship. Unfortunately, the importance of this factor is difficult to assess because no records were kept of the parents of the offspring at the time of release and our sample in 1994 included individuals hatched up to 23 years previously. In any case, increasing the reproductive success of individuals currently contributing little to the repatriated gene pool should probably be encouraged because: (i) even if some of their genes impart low fitness, genetic variation at other unlinked loci might be beneficial for individuals in following generations; and (ii) high reproductive success in captivity is not necessarily correlated with fitness in the wild.
Utility of these results

Further analyses:
(1) a contamination in the Española program
Here we present a unique example of the use of molecular conservation genetics to detect genetic contamination of a captive breeding-repatriation program of a once critically endangered species: the Española giant tortoise (Galápagos).
The genetically distinct population of giant Galápagos tortoises (Geochelone hoodensis, at times considered a subspecies of G. nigra) occupying the island of Española in the extreme southeastern region of the Galápagos archipelago was in grave danger of extinction in the late 1960s due to hunting activities from sealing, whaling, and pirate ships, as well as habitat destruction by feral goats. Thanks to the captive-breeding and reintroduction program initiated by the Charles Darwin Research Station (CDRS) and Galápagos National Park (PNG) in late 1960s (see above), more than 2000 offspring have been repatriated to date, the island has undergone significant ecological recuperation, repatriated tortoises now reproduce in situ, and the core population of 15 parents continues to reproduce in captivity at the Breeding Centre.
Our previous analyses (see above) of 15 informative microsatellite loci allowed us to determine the captive parents of 118 surviving released individuals collected in 1994; at that time, this represented about 40% of the repatriated population. This analysis indicated that contributions of the 15 breeders are highly skewed, reducing the effective population size (Ne) to an alarming low value of 5.7. Estimating that Ne for the current repatriated population is 1200 (based on a 60% survival rate of released offspring and on the optimistic assumption that sex ratio and reproductive success are not biased on the island), Ne over the two generations would only reach 11.3 (i.e., the harmonic mean between the parental and offspring generation, see above). In other words, the current orchestration of the breeding program generated inbreeding that reduced genetic variation of the current population of about 1200 individuals to a level equivalent to that expected for, at best, a population of 11 unrelated individuals. In order to fine-tune the estimate of Ne and analyse its evolution through time, we collected in 2003 and 2004 blood samples from 316 additional tortoises on Española.
Surprisingly, one individual (E1465) sampled on Española exhibits one alien allele (i.e, not found in any of the 15 captive parents) at 8 of the 15 loci investigated. It is therefore impossible that both parents of this individual are among the 15 breeders. Sequencing of a section of the control region in individual E1465 reveals the single haplotype identified previously as specific to the Española population. These results suggest that E1465 is a hybrid between a female Española tortoise and a male from another island. To assess the geographic origin of the father, we genotyped E1465 at 9 loci that had been previously used for analysing genetic variability and population structure of giant tortoises across the whole archipelago. The "alien" alleles revealed for E1465 are found at varying frequencies in populations throughout Isabela, Santiago, Pinzón, Santa Cruz, and San Cristóbal. Using a genotypic database of 304 field-sampled individuals from all populations on the major islands, we performed assignment tests using two methods. Both methods assigned E1465 with high probabilities to the Española and Pinzón island populations.
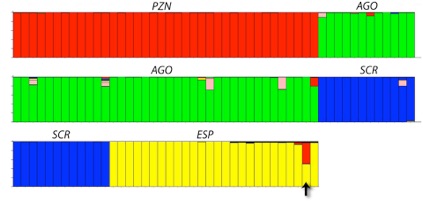
Figure: Inferred ancestry of individuals from the islands of Pinzón (PZN), Santiago (AGO), Santa Cruz (SCR), and Española (ESP) according to STRUCTURE analysis (see references in original publication). Membership coefficients are colour-coded according to islands: red (Pinzón), green (Santiago), blue (Santa Cruz), and yellow (Española). E1465 (arrow) exhibits membership coefficients of 0.501 and 0.461 for Española and Pinzón, respectively.
The most likely explanation is that E1465 is a hybrid between an Española female and a Pinzón male. Inspection of the genotype of E1465 indicates that none of the 12 captive females can be its mother (nor any of the three captive males can be its father), hence, the hybridization event must have taken place on Española (rather than at the captive breeding centre). The cause for the (past or current) presence of a Pinzón male on Española is unknown but is most likely linked to human transport. One possibility is the Pinzón male was a transplant due to the extensive exploitation and sometimes translocation of tortoises by 17–19th century whaling and other activities. It is conceivable that such an animal was missed in the attempts to find all remaining tortoises on Española in 1965. Such transplants have been detected on other islands. This would imply Pinzón genetic contamination should have been occurring for several generations, a possibility for which there is no evidence. Indeed, the fact that only a single such hybrid offspring has been found in a sample of 400+ survivors on Española (present data plus those in the previous paper, see above) is strong evidence against long-term contamination. A more likely scenario is that early in the Española releases (early 1970s), a Pinzón male was accidentally incorporated into the repatriates. A captive breeding program of Pinzón tortoises at the captive breeding centre predates the Española program, so Pinzón juvenile tortoises were present there at the time of release. Furthermore, it is noteworthy that morphologies of Pinzón and Española tortoises, especially as juveniles, are largely indistinguishable.
Clearly, if one wishes to maintain the guideline/principle of restoring "natural" populations, it is important to remove from Española the individuals that compromise the integrity of the Española gene pool. In addition to E1465, we also need to search for the possible presence of its father and (half-) siblings. Note that we cannot rule out the possibility that the contaminating Pinzón individual may be a grand-parent of E1465. However, given that our estimate based on size is that E1465 is minimum 9 years old, and tortoises reach sexual maturity at about 20 years of age, it is impossible that E1465 is a grandchild of a male released within the last 35 years. Finding E1465 should be relatively easy because we marked it with a permanent Passive Integrated Transponder tag, as is the case for all individuals that we sampled on the island. Finding the father and its descendants will be more difficult, because they do not carry any tag, as their blood has never been sampled. We will therefore need to search for unmarked tortoises, sample them, and use a diagnostic field-based test that will allow us to differentiate Pinzón vs Española alleles.
Regardless of the means by which Pinzón alleles have entered the Española lineage, our analysis (i) indicates that the rate of contamination of the Española breeding repatriation program is very low, and (ii) underlines the utility of the approach used by the GNP and the CDRS, i.e., use molecular genetic approaches to monitor the breeding-repatriation program. One future step might be to routinely perform a diagnostic PCR-based test on all the tortoises that will be released in the future to assess their correspondence with the target population. Despite this detected single contamination, it is highly noteworthy to emphasize the success of this repatriation program conducted by the PNG and CDRS over nearly 40 years in difficult conditions and involving release of over 2000 captive-bred tortoises that now reproduce in situ. The recent incorporation of molecular genetic analysis of the program is providing further insights and guidance that will aid in assuring the survival of this unique linage of a spectacular animal.
Further analyses:
(2) comparing differential reproductive success and survival in the Española repatriation program
In Progress.
Further analyses:
(3) Growth Patterns in repatriated Española tortoises
In Progress.
